























 |
 |
 |
|
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
||
 |
 |
|
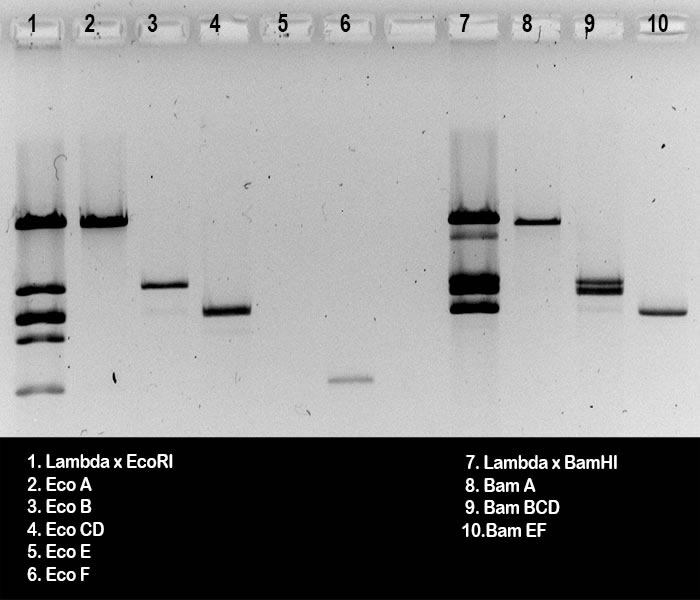
This is a black-and-white photograph of the gel, used
to check the DNA extractions from gel slices. The gel was run by
groups B from the wet lab. Lanes 2 - 6 (A) and lanes 8 - 10 (B) show 10 ml samples from DNA extraction preps, after 2 hours electrophoresis. DNA size markers to compare with the extracted DNA fragments were run in lanes 1 and 7; they show EcoRI and BamHI digests, 0.5 μg Lambda DNA each.
It is clear that in lane 2, fragment EcoA is present
(C). In lane 8 BamA is visible (H), a mixture of BamB, BamC and BamD in lane 9 (I), and a mixture of BamE and BamF in lane 10 (J).
Some contaminations are detected: (K): Marker digests for lanes 1 and 7 were not heated prior to loading in the gel slots, so there must have been some EcoAF cos-site containing DNA in lane 1 (L); and for the same reason the BamCF cos-site containing band is visible in lane 7 (M).
To estimate (as an example) the DNA concentration in
the EcoB prep, of which 10μl was run in lane 3, you could do the
following:
|