|
Blotting |
|||||
|
|
|
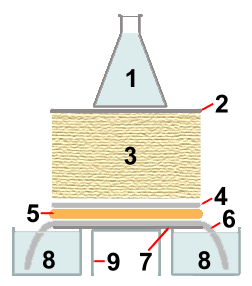 |
for 8 and 9, one large open container with a small plate on top, may be used |
During the capillary transfer of 0.4M NaOH from containers 8
into the paper towels 3, the DNA is denatured (made
single-stranded) by the high pH of the transfer solution, and it is
bound to one side of the membrane sheet 4. The binding
is covalent when positively charged nylon is used. Uncharged nylon needs
to be "baked" at 80 °C or treated with UV light, after the blotting
step, to make the DNA binding covalent. Small DNA fragments are
efficiently transferred in about 1 hour; fragments longer than 15,000
base pairs require an overnight transfer and, even then, may not be
completely transferred.
After the blotting using 0.4 M NaOH, the DNA bands have bound to the
nylon sheet, the EthBr has been washed out of the gel and into the paper
towels, and the gel is flattened to form a very sturdy gel.
If you would like to check the successful transfer of the DNA bands,
you should incubate the resulting gel for at least one hour in a large
volume of TAE buffer with EthBr. Use a UV lightbox to check for any
remaining bands in the gel.
note:
Additional safety precautions
(lab coat, safety glasses) should be taken for handling 0.4 M NaOH
solutions
|
|
See also the Movie about Blotting Read the Movie Text |


